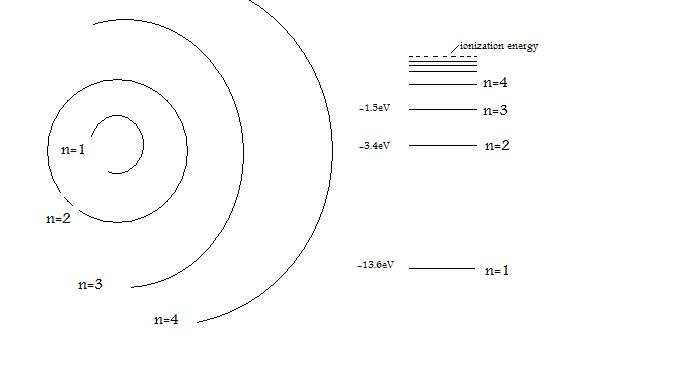
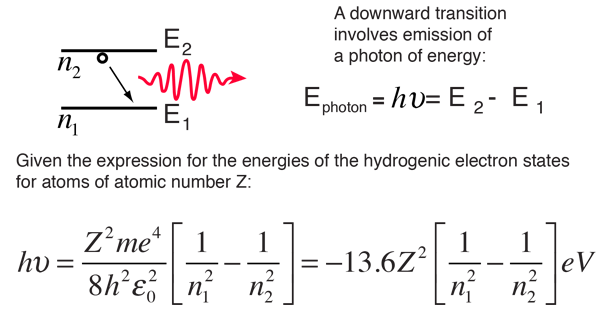
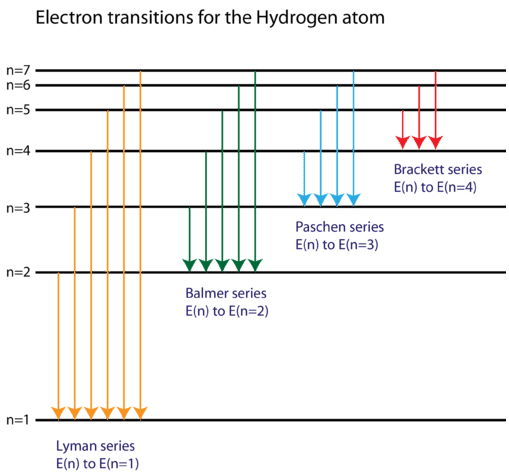
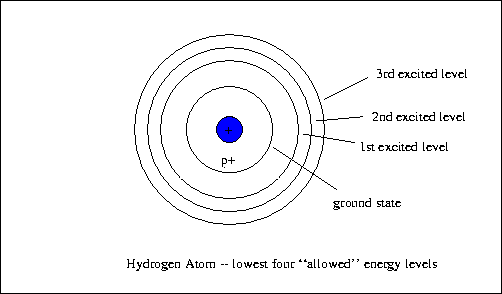
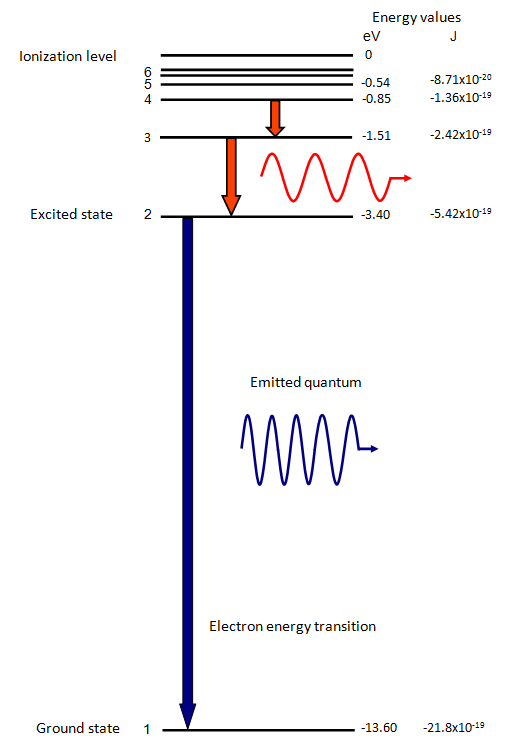
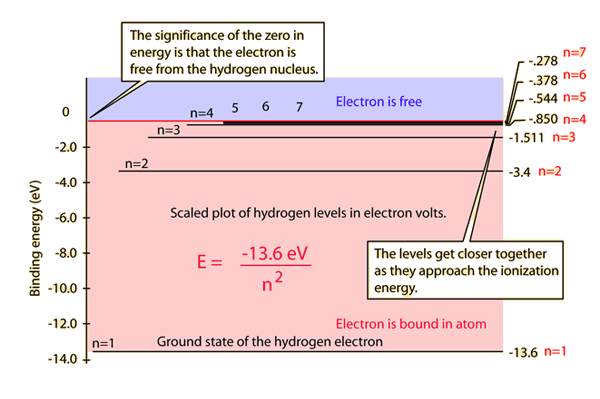
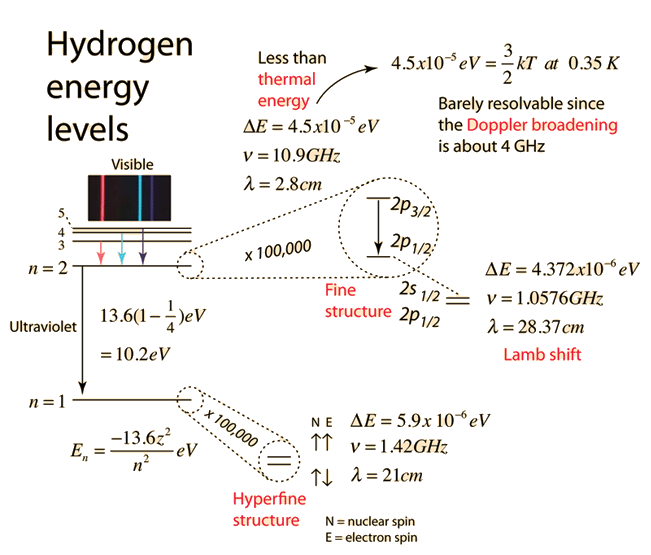The energy of the electron in the ground state of hydrogen atom is - 13.6 eV. Find the kinetic energy and potential energy of electron in this state.
The electron energy in hydrogen atom is given by En = -217 x 10^-12/n^2 ergs. - Sarthaks eConnect | Largest Online Education Community

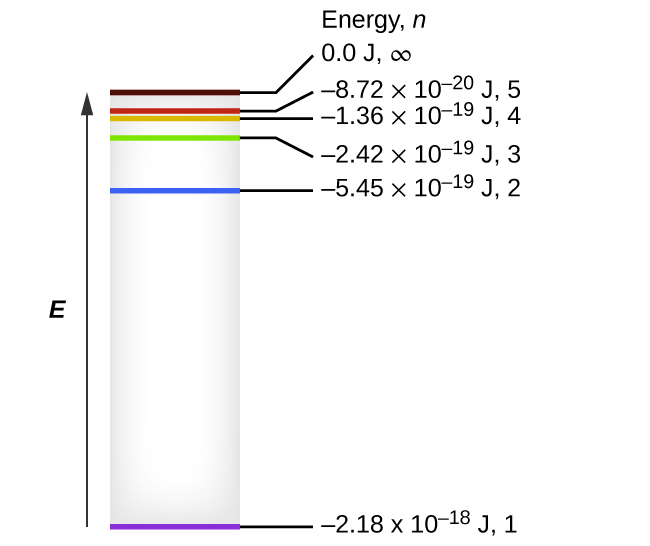
The electron energy of hydrogen atom in the ground state works out to be - 2.18 xx 10^(-18) J per atom. Calcu
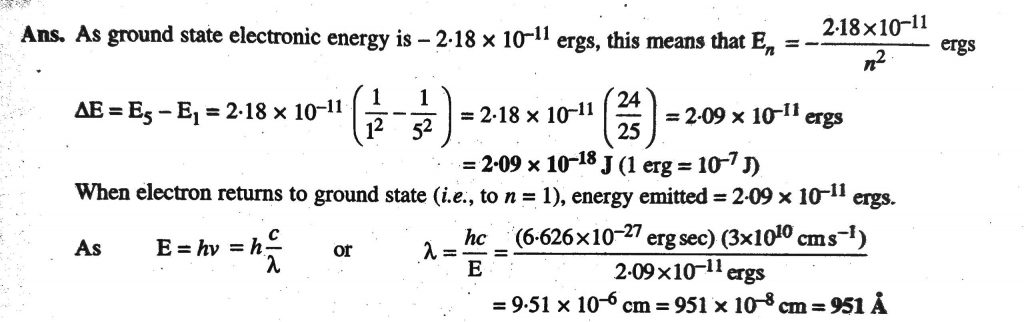
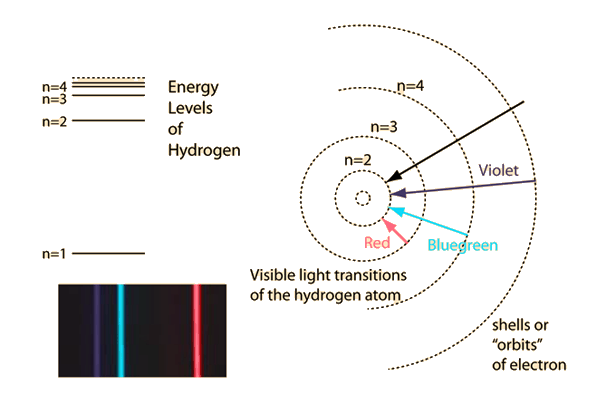
What is the energy in joules required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit ? And what is the wavelength of

According to Bohr's theory, the electronic energy of hydrogen atom in the Bohr's orbit is given byE n = 21.76 × 10 ^-19/n^2 JCalculate the longest wavelength of light will be needed

The electron energy in hydrogen atom is given by En = ( - 2.18 × 10^-18) n^2 joules.Calculate the energy required to remove an electron completely from the n = 2 orbit.
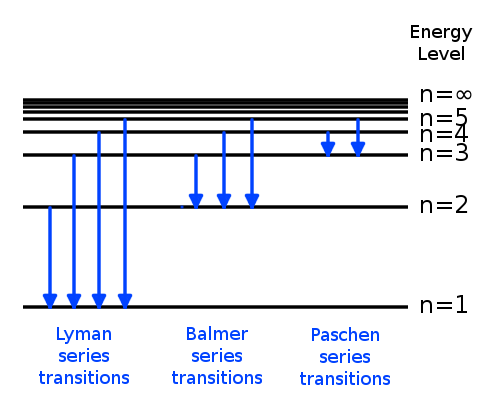
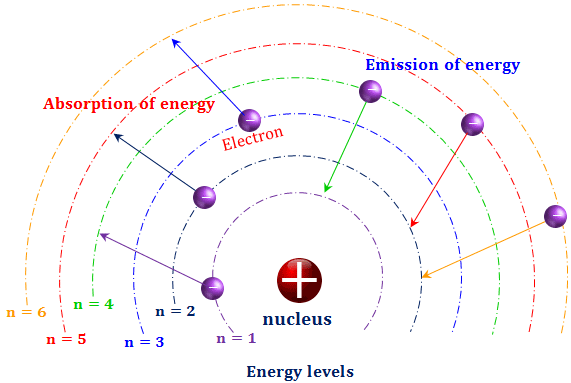
How can an electron leap between atomic levels without passing through all the space in between? | Science Questions with Surprising Answers